Cell-Free Protein Expression
VectorBuilder provides high-quality protein production service through our Cell-Free Protein Synthesis (CFPS) platform. CFPS enables in vitro protein expression outside of living cells. The process of recombinant protein expression through the CFPS has been optimized and streamlined, allowing for completion in as little as five days. This significant acceleration enhances the efficiency of your workflow.
Highlights
- Fast turnaround time
- High level of control over the reaction conditions
- Suitable for high-throughput production of a range of target molecules
- Adaptable for producing antibody fragments that are difficult to express in cellular systems
Service Details
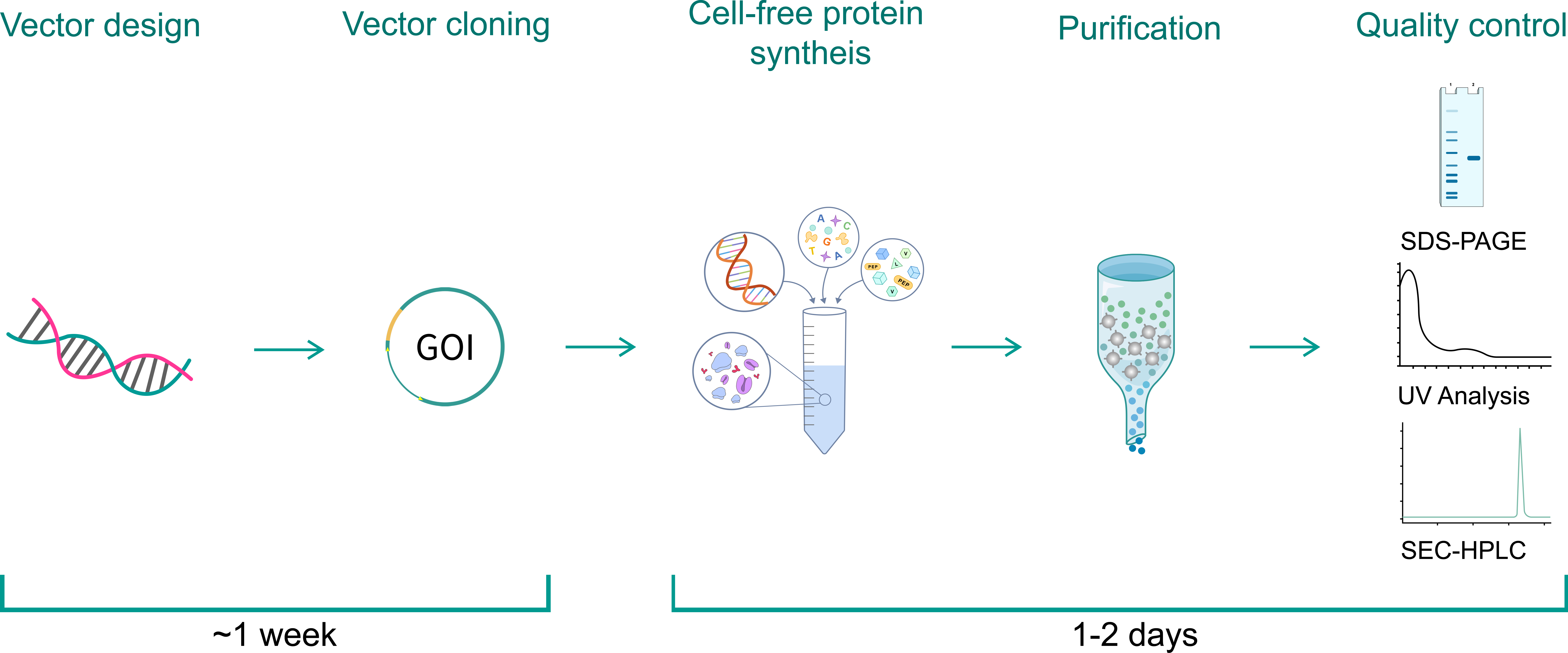
Our workflow starts from the design and construction of the expression vector, which will be used as the template in the CFPS. E. coli cells are lysed, and the crude extracts are collected from cultured cells. The lysate, which contains the necessary cellular machinery, will be further processed, and necessary components such as energy molecules, amino acids, and DNA or mRNA template will be supplemented to initiate transcription and translation for protein synthesis. The process of cell-free synthesis, protein purification, and QC can be finished in as fast as 1 day.
Shipping and storage
Most of our custom recombinant proteins are delivered as frozen liquid on dry ice. Upon receipt, they should be stored at -20°C to -80°C under sterile conditions. Recombinant proteins typically remain stable for up to a year if stored properly. Additionally, we recommend aliquoting the recombinant proteins upon receiving and avoiding freeze-thaw cycles.
Quality control (QC)
All vectors cloned by VectorBuilder come with a 100% sequence guarantee. All recombinant proteins produced by VectorBuilder undergo stringent quality control. Default QC for most systems includes 1) validation of the protein expression vector by restriction digestion analysis and Sanger sequencing; and 2) determination of protein concentration and purity by A260/280 measurement and SDS-PAGE. Common additional QC services are shown in the table below, which can be provided upon request.
| Additional QC services | Method |
|---|---|
| Endotoxin test | LAL |
| Protein characterization | Western Blot |
| Intact MS (reduced) | |
| SEC-HPLC | |
| SEC-MALs | |
| Protein N-terminal sequencing | |
| Host cell protein test | |
| Tag removal | Protease digestion |
| Kinetics and affinity analysis | Octet |
| Biacore | |
| Pathogen testing panel for rodents | |
Technical Information
Case study
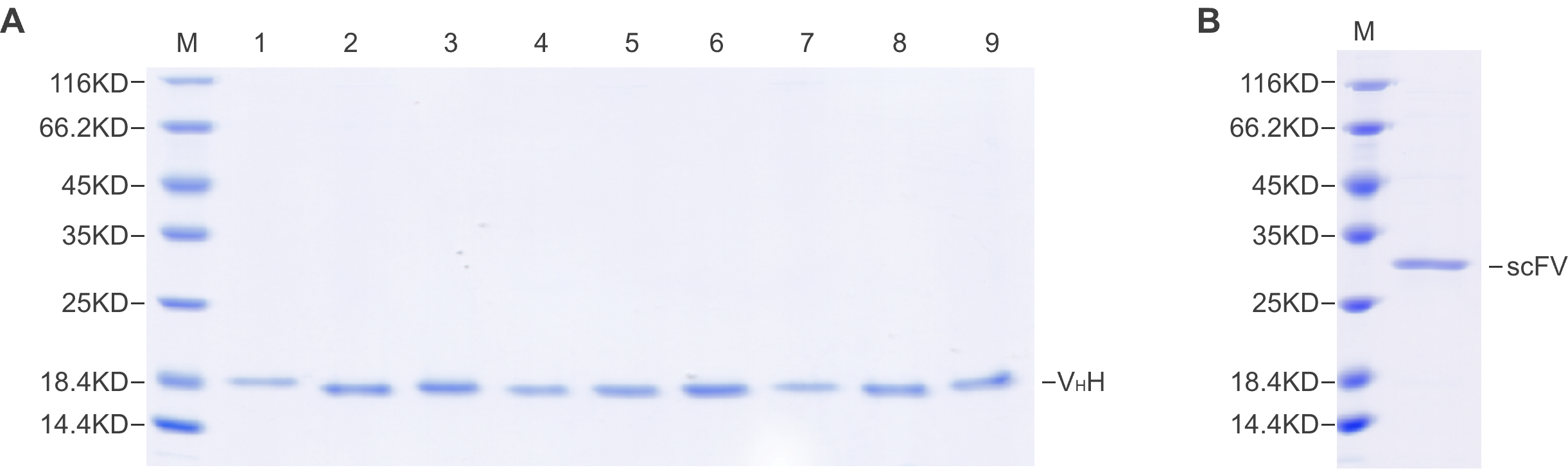
Figure 1. (A) High-throughput VHH synthesis by CFPS. The purity was determined to be >90% by SDS-PAGE. (B) scFv synthesis by CFPS. The purity was determined to be >90% by SDS-PAGE.
How to Order
Customer-supplied vectors
If customer-supplied materials are needed, please send them to us following the Materials Submission Guidelines. Please strictly follow our guidelines to set up shipment to avoid any delay or damage of the materials. All customer-supplied materials undergo mandatory QC by VectorBuilder, which may incur $100 surcharge for each item. Please note that production may not be initiated until customer-supplied materials pass QC.
FAQ
All recombinant protein expression systems have their pros and cons which should be taken into consideration while selecting the optimal system for your project. The table below summarizes the pros and cons of each system.
| Recombinant protein expression system | Pros | Cons |
|---|---|---|
| Bacterial |
|
|
| Mammalian cells |
|
|
| Insect |
|
|
| Cell-free system |
|
|
Tags are frequently utilized for recombinant protein production. They streamline the purification process, and certain tags have demonstrated improvement in protein solubility, yield, or purity. If the tag is attached to the protease cleavage site, it can be removed after purification, and the efficiency of cleavage varies depending on the target protein. The careful selection of an appropriate tag is crucial for downstream protein expression and purification. The table below provides an overview of commonly used tags along with their advantages and limitations.
| Tag | Commonly applied system | Advantages | Limitations |
|---|---|---|---|
| GST | Bacteria, insect |
|
|
| His | All |
|
|
| SUMO | Bacteria, insect |
|
|
| Flag | Mammalian cells, insect |
|
|
| MBP | Bacteria, insect |
|
|
| Fc | Mammalian cells, insect |
|
|
For more information regarding tags, please visit protein tags.
Yes, CFPS involves mixing a cell extract containing the necessary machinery for protein synthesis with other essential components. By incorporating components derived from eukaryotes, the synthesis machinery closely resembles that in eukaryotic cells, enabling the accurate synthesis of eukaryotic proteins.




