Bacterial Protein Expression
E. coli is the most widely used host for recombinant protein production due to the several advantages it offers, including short production time, technical simplicity, scalability, and low cost. VectorBuilder specializes in the production of recombinant proteins in E. coli with a highly optimized workflow for the expression of cytoplasmic and periplasmic as well as soluble and insoluble proteins.
Highlights
- Low cost, high yield, and easy to scale up
- Optimized strategies for both soluble protein and inclusion bodies
- Various vector backbones and bacterial strains are available for maximum optimization
Service Details
Price and turnaround Price Match
| Service Module | Brief Description | Deliverables | Price (USD) | Turnaround |
|---|---|---|---|---|
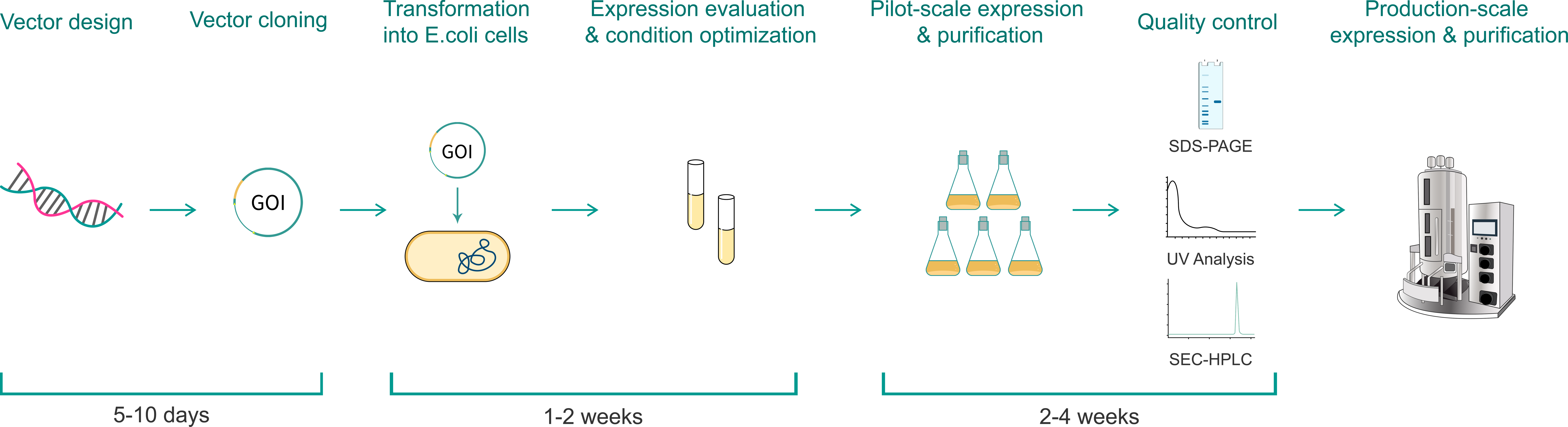
| Vector design and cloning | Vector design includes fusion partner selection, purification tag selection, and codon optimization. This is followed by gene synthesis and cloning into the bacterial recombinant protein expression vector, such as the pET. | E. coli glycerol stock | From $299 | 5-10 days |
| Expression evaluation and condition optimization | The expression vector is transformed into the appropriate host strain, such as BL21 (DE3). A small-batch evaluation will be conducted with various conditions, and the expression and solubility of the target protein will be assessed *. | Expression evaluation report | From $600 | 1-2 weeks |
| Pilot-scale expression and purification | Based on the evaluation from the last step, 0.5-1 mg of purified target proteins ** can be produced if feasible, and quality control (QC) will be performed. |
|
From $1,200 | 2-4 weeks |
| Production-scale expression and purification | Scaled up production of >100 mg of purified proteins. |
|
Please inquire | |
* If proteins form inclusion bodies, they will undergo solubilization, followed by refolding and purification. The appropriate refolding strategies will be determined based on the sequence and structural properties of the protein. The pricing will be adjusted accordingly.
** Purification methods will be determined based on the construct design and protein characteristics.
Shipping and storage
The default deliverable for vector cloning is E. coli glycerol stock. Upon request, small amounts of plasmid DNA can be provided if available without additional charge. Most of our recombinant proteins are delivered as frozen liquid on dry ice. Upon receipt, they should be stored at -20°C to -80°C under sterile conditions. Recombinant proteins typically remain stable for up to a year if stored properly. Additionally, we recommend aliquoting the recombinant proteins upon receiving and avoiding freeze-thaw cycles.
Quality control (QC)
All vectors cloned by VectorBuilder come with a 100% sequence guarantee. All recombinant proteins produced by VectorBuilder undergo stringent quality control. Default QC for most systems includes 1) validation of the protein expression vector by restriction digestion analysis and Sanger sequencing; and 2) determination of protein concentration and purity by A260/280 measurement and SDS-PAGE. Common additional QC services are shown in the table below, which can be provided upon request.
| Additional QC services | Method |
|---|---|
| Endotoxin test | LAL |
| Protein characterization | Western Blot |
| Intact MS (reduced) | |
| SEC-HPLC | |
| SEC-MALs | |
| Protein N-terminal sequencing | |
| Host cell protein test | |
| Tag removal | Protease digestion |
| Kinetics and affinity analysis | Octet |
| Biacore | |
| Pathogen testing panel for rodents | |
Technical Information
Vector system
Common vector systems for bacterial recombinant protein expression include pET, pBAD, pCS, etc. Among them, the pET vector stands out as a powerful and widely used system. The gene of interest is cloned into the pET vector under the control of the strong bacteriophage T7 transcription and translation regulatory system. Expression is activated by providing T7 RNA polymerase within the cell. When the system is fully induced, nearly all the cell's resources are devoted to expressing the gene of interest. The map below contains the key components of the pET recombinant protein expression vector.
For more information regarding VectorBuilder's bacterial protein expression vector system, please visit our vector guides.
Figure 1. Map of a pET bacterial recombinant protein expression vector.
Case study
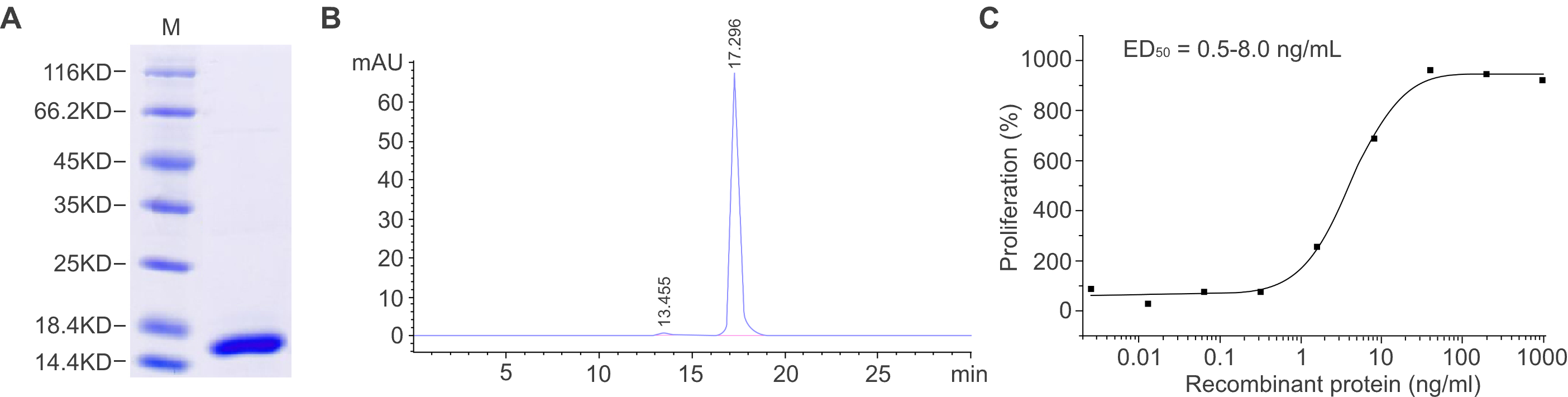
Figure 2. Characterization of a recombinant protein produced with the E. coli system. (A) SDS-PAGE analysis shows the molecular mass of the recombinant protein, and the purity was determined to be ≥ 95%. (B) The purity was determined to be ≥ 95% by SEC-HPLC. (C) The biological activity of the recombinant protein was measured by a cell proliferation assay. The ED50 is between 0.5 and 8 ng/ml.
How to Order
Customer-supplied vectors
If customer-supplied materials are needed, please send them to us following the Materials Submission Guidelines. Please strictly follow our guidelines to set up shipment to avoid any delay or damage of the materials. All customer-supplied materials undergo mandatory QC by VectorBuilder, which may incur $100 surcharge for each item. Please note that production may not be initiated until customer-supplied materials pass QC.
FAQ
All recombinant protein expression systems have their pros and cons which should be taken into consideration while selecting the optimal system for your project. The table below summarizes the pros and cons of each system.
| Recombinant protein expression system | Pros | Cons |
|---|---|---|
| Bacteria |
|
|
| Mammalian cells |
|
|
| Insect |
|
|
| Cell-free system |
|
|
Tags are frequently utilized for recombinant protein production. They streamline the purification process, and certain tags have demonstrated improvement in protein solubility, yield, or purity. If the tag is attached to the protease cleavage site, it can be removed after purification, and the efficiency of cleavage varies depending on the target protein. The careful selection of an appropriate tag is crucial for downstream protein expression and purification. The table below provides an overview of commonly used tags along with their advantages and limitations.
| Tag | Commonly applied system | Advantages | Limitations |
|---|---|---|---|
| GST | Bacteria, insect |
|
|
| His | All |
|
|
| SUMO | Bacteria, insect |
|
|
| Flag | Mammalian cells, insect |
|
|
| MBP | Bacteria, insect |
|
|
| Fc | Mammalian cells, insect |
|
|
For more information regarding tags, please visit protein tags.
Described below are some common reasons why you might be having issues expressing your recombinant protein in bacteria.
The plasmid is placed in an inappropriate host strain.
The plasmid may not be expressed in the inappropriate host strain. Most vectors from VectorBuilder are shipped as E. coli stock in the cloning host strain VB UltraStable™. VB UltraStable™ is the preferred cloning host due to its ability to maintain the stability of the plasmid, but it may not be suitable for recombinant protein expression. For recombinant protein production, we recommend transferring the vector to BL21(DE3) or HMS174(DE3) host bacteria strains, which carry chromosomal copies of the T7 RNA polymerase gene driven by the LacUV5 promoter. In cases when the toxicity of the gene of interest is an issue in these expression host strains, the use of hosts carrying the pLysS or pLysE plasmids may be beneficial. These plasmids suppress basal expression of the gene of interest by producing T7 lysozyme, a T7 RNA polymerase inhibitor.
The protein expressed on the vector is “problematic”.
Sometimes when the protein being expressed is insoluble, misfolded, improperly cleaved, or toxic to the bacterial host, it may result in poor induction of the recombinant protein. In this case, you will need to optimize your induction system (see below) or express your gene of interest in a more tolerant host strain or from an alternative expression system.
The induction system is not optimal.
Depending on the gene to be expressed, the expression vector, and the host strain, you may need to consider optimizing various factors in your induction system. These include OD600 (usually between 0.6 and 0.8), the concentration of the inducing agent (e.g., IPTG or L-arabinose), the duration of induction, and the induction temperature. It is particularly important to optimize the induction temperature when dealing with 'problematic' proteins, and different temperatures can be tested, such as 16°C, 25°C, 30°C, and 37°C. In rare cases, for unknown reasons, different clones of the same expression vector may exhibit different induction behaviors. Therefore, it may be necessary to pick several single colonies to test individually and select the one that shows optimal induction performance.
To circumvent the issues mentioned earlier and save your valuable time for actual research, consider outsourcing your recombinant protein expression needs to VectorBuilder.