Virus Packaging Plasmids

VectorBuilder offers GMP-like and GMP-grade lentivirus and AAV packaging plasmids for the manufacturing of pre-clinical and clinical viral vectors for gene and cell therapies. Produced in our state-of-the-art facilities, each plasmid has been optimized to produce high titers of virus and has undergone thorough quality control.
Plasmids offered
We offer the following GMP-like and GMP-grade plasmids:
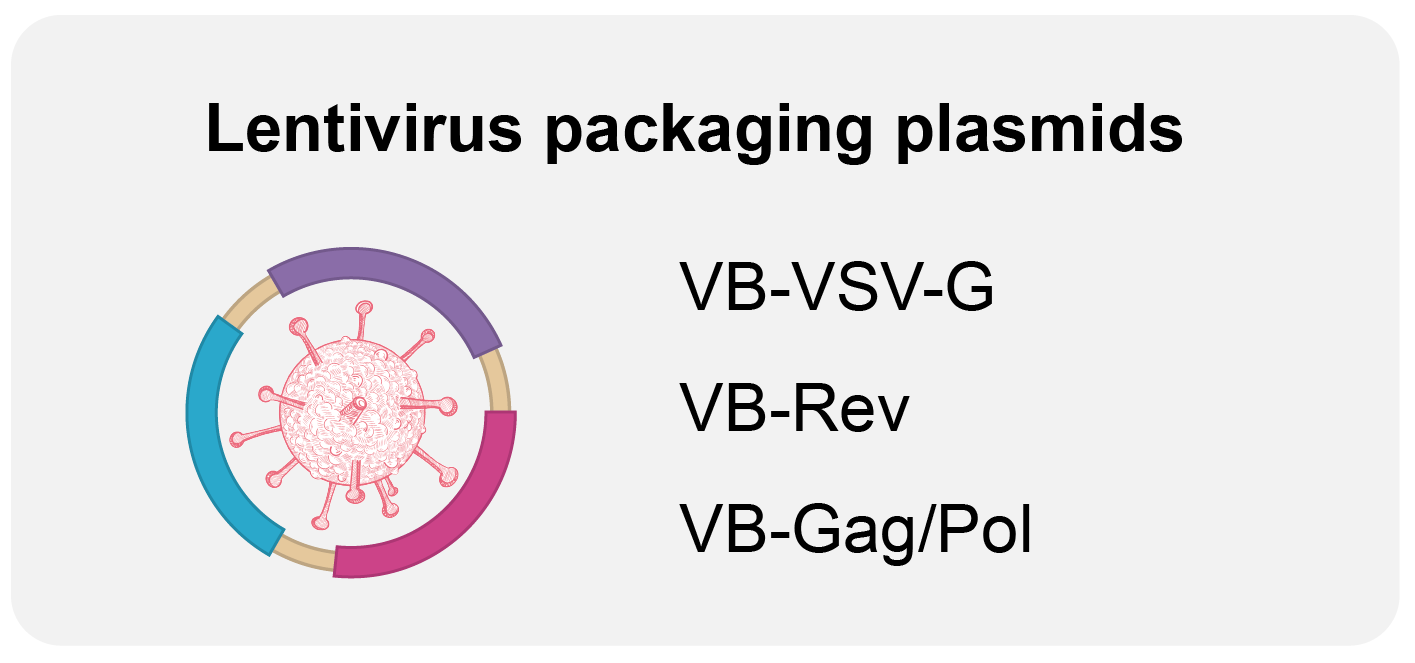
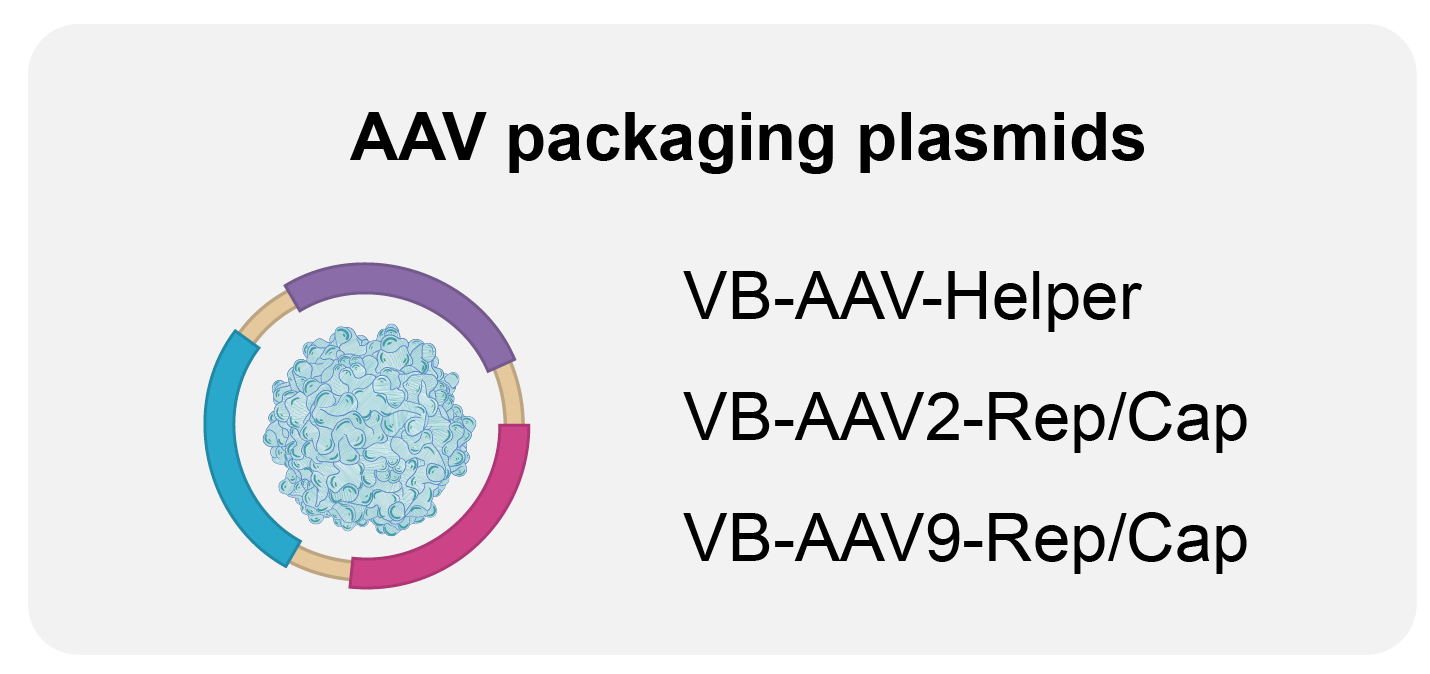
Highlights
- Optimized and validated for high virus yield
- Off-the-shelf plasmids available for immediate use
- Plasmids produced under animal-free and antibiotic-free conditions
- Royalty free
- DMFs available simplifying FDA IND application
- Kan resistance
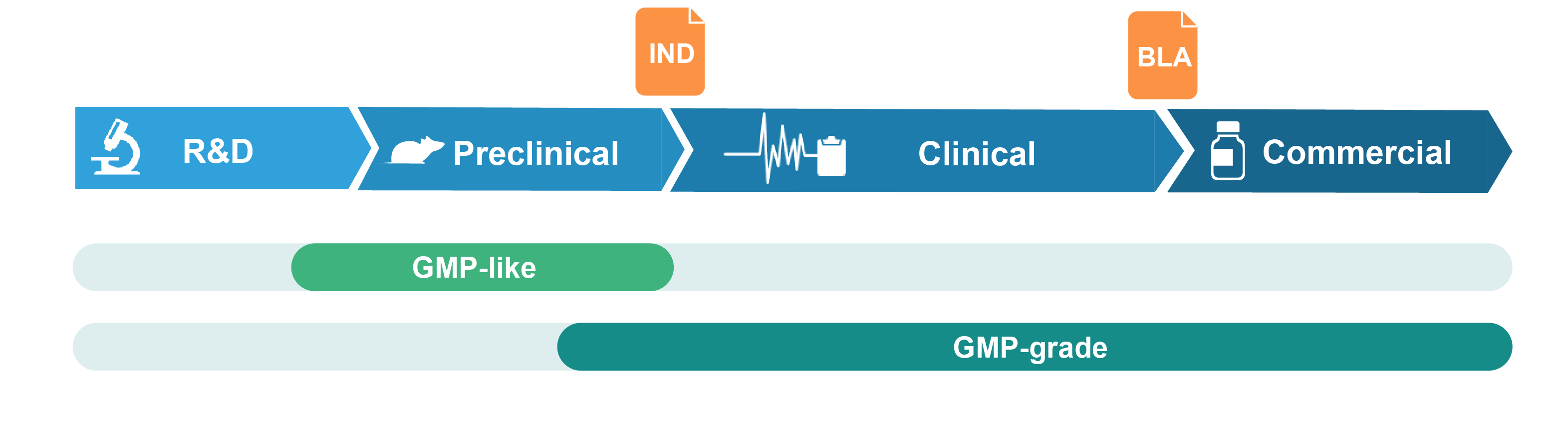
Grades of Packaging Plasmid Offered
-
GMP-like plasmid
GMP-like plasmid DNA is intended for pre-clinical studies such as animal testing of drug safety and metabolism. It is produced in a manner that adopts key features of GMP guidelines, including comparable production process and similar quality attributes. Production is performed in segregated production suites with document control and traceability. GMP-like grade can thus be viewed as a small-scale mimic of the final GMP product, but with much lower cost and faster timeline. A certificate of analysis (COA) and TSE/BSE statements are provided at product release.
-
GMP-grade plasmid
GMP-grade plasmid DNA is produced in our certified GMP suite with strict adherence to GMP guidelines. A comprehensive quality assurance system is implemented throughout the production process. A wide range of in-process and release QC assays are performed to ensure that the plasmid DNA meets or exceeds the desired quality and safety standards. A batch release report fully documenting the production process and a COA are provided at product release.
Learn more about our GMP plasmid manufacturing
| Product | Quantity | GMP-like | GMP-grade |
|---|---|---|---|
|
5 mg | Please inquire | Please inquire |
| 10 mg |
Quality Control and Release Criteria
The list below summarizes the main QC assays and specifications for the release of GMP-like and GMP-grade plasmid.
| QC | Method | GMP-like | GMP-grade |
|---|---|---|---|
| Plasmid concentration | UV spectrophotometry | ≥500 ug/ml | ≥500 ug/ml |
| Restriction digestion | Agarose gel electrophoresis | Identical to expected restriction pattern | Identical to expected restriction pattern |
| Sanger sequencing | Sanger sequencing of the entire plasmid | Identical to reference sequence | Identical to reference sequence |
| A260/A280 | UV spectrophotometry | 1.80-2.00 | 1.80-2.00 |
| ccc plasmid DNA ratio | EtBr stained agarose gel electrophoresis | ≥80% | ≥80% |
| Residual protein | BCA or equivalent | ≤2.00% | ≤2.00% |
| Residual RNA | Fluorescence analysis or equivalent | ≤5.00% | ≤5.00% |
| Residual host cell DNA | Quantitative PCR or equivalent | Report result | ≤5.00% |
| Endotoxin | Kinetic chromogenic assay | ≤50 EU/ml | ≤10 EU/ml |
| Sterility | Direct inoculation | No growth | No growth |
Documents
Brochures & Flyers



